Our patient profiles provide some key features to look for when making prescribing decisions.

Part-time student and waiter

general labourer

full-time legal clerk

avid social media user

High school student with part-time job

active child

active child
* Individual patient cases. May not be representative of the general population.
Adverse
Events Reported by ≥ 1% Children (6-12 years):
6 Week Open-Label Titration Phase Followed by a 1-Week Double-Blind Treatment Phase1‡
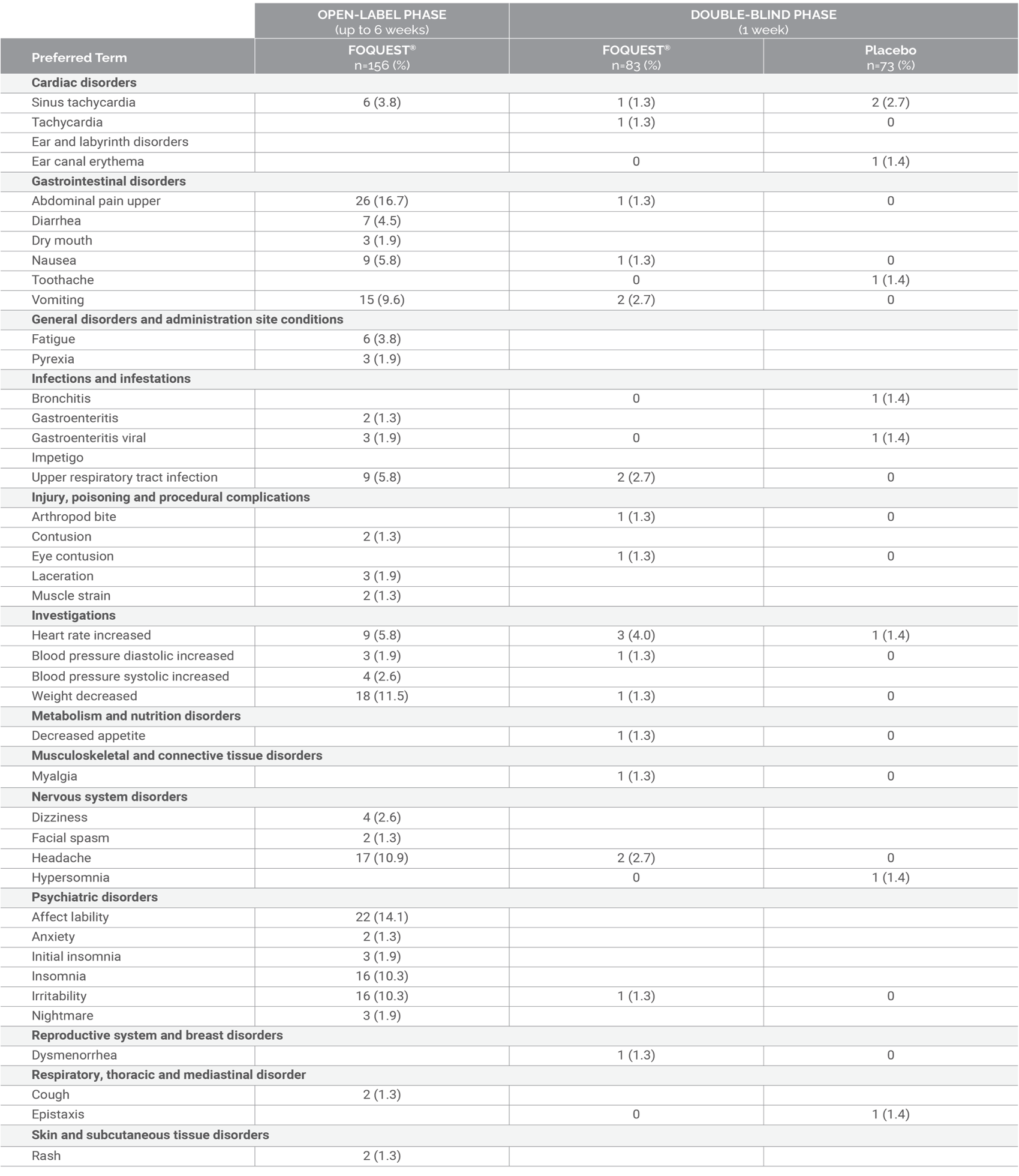
Adapted from FOQUEST® product monograph.1
‡ Open-label phase: All participants started at 25 mg/day. Doses were titrated each week to the next highest
dose according to tolerability: 25, 35, 45, 55, 70 and 85 mg/day. Participants may have returned to a lower dose
if necessary. When optimal dose was reached, participants entered double-blind, placebo-controlled phase (half
of the participants were randomized to receive placebo while the other half remained on their optimized dose).
The maximum daily dose for children and adolescents (6 to
<18 years old) is 70 mg.
Adverse Events Reported by ≥ 1% Adults (≥18 years): Pivotal Trial1¶
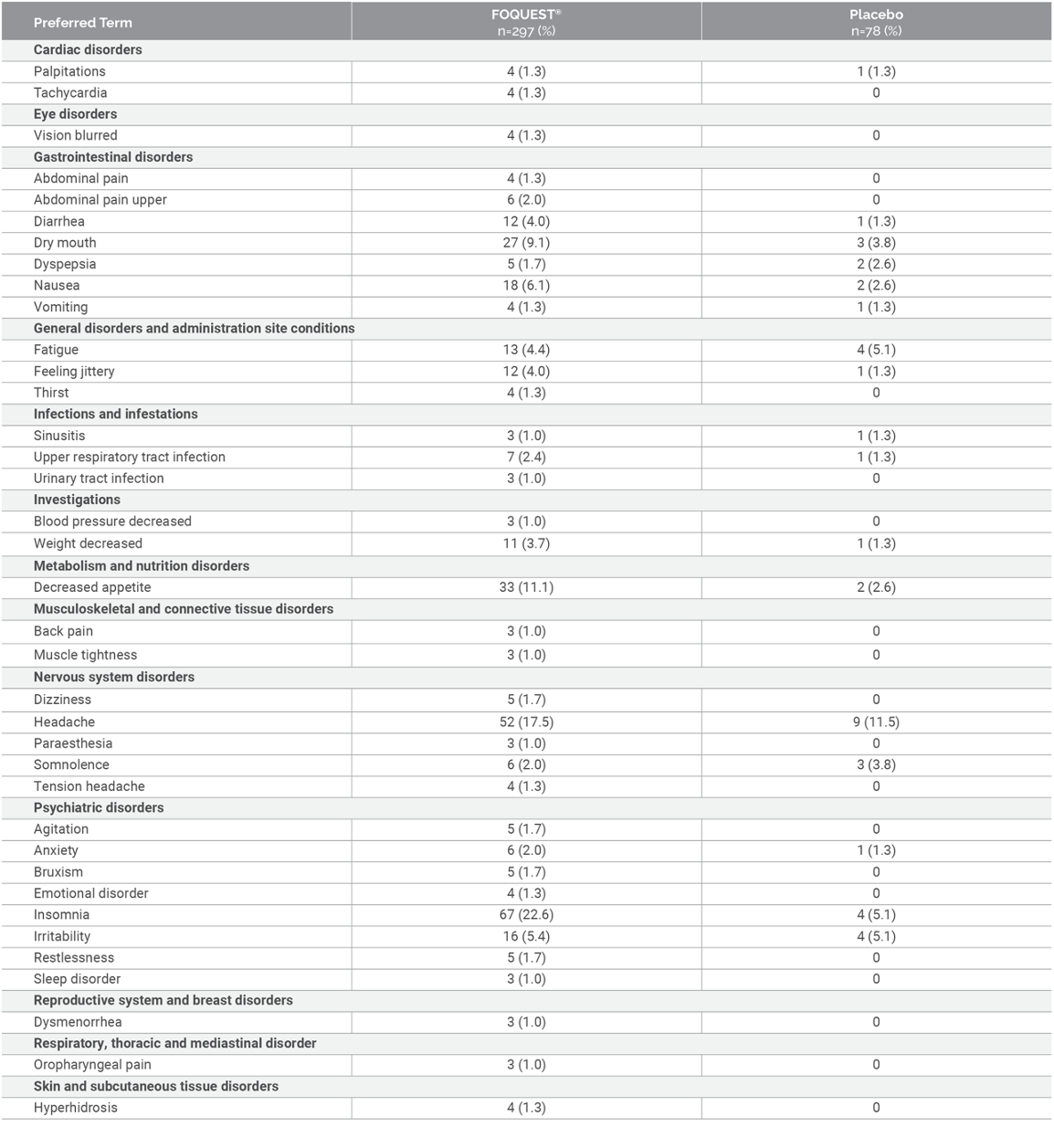
Adapted from FOQUEST® product monograph.1
¶ Study duration: 4 weeks; Doses: 25, 45, 70 and 100 mg/day. The maximum daily dose for adults (≥18 years) is
100 mg.
Adverse Events Reported by ≥1% Adults (≥18 years): Post-Market Trial (Up to 7 weeks Open-Label
Titration
Phase followed by a 1 Week Double-Blind Treatment Phase)1//
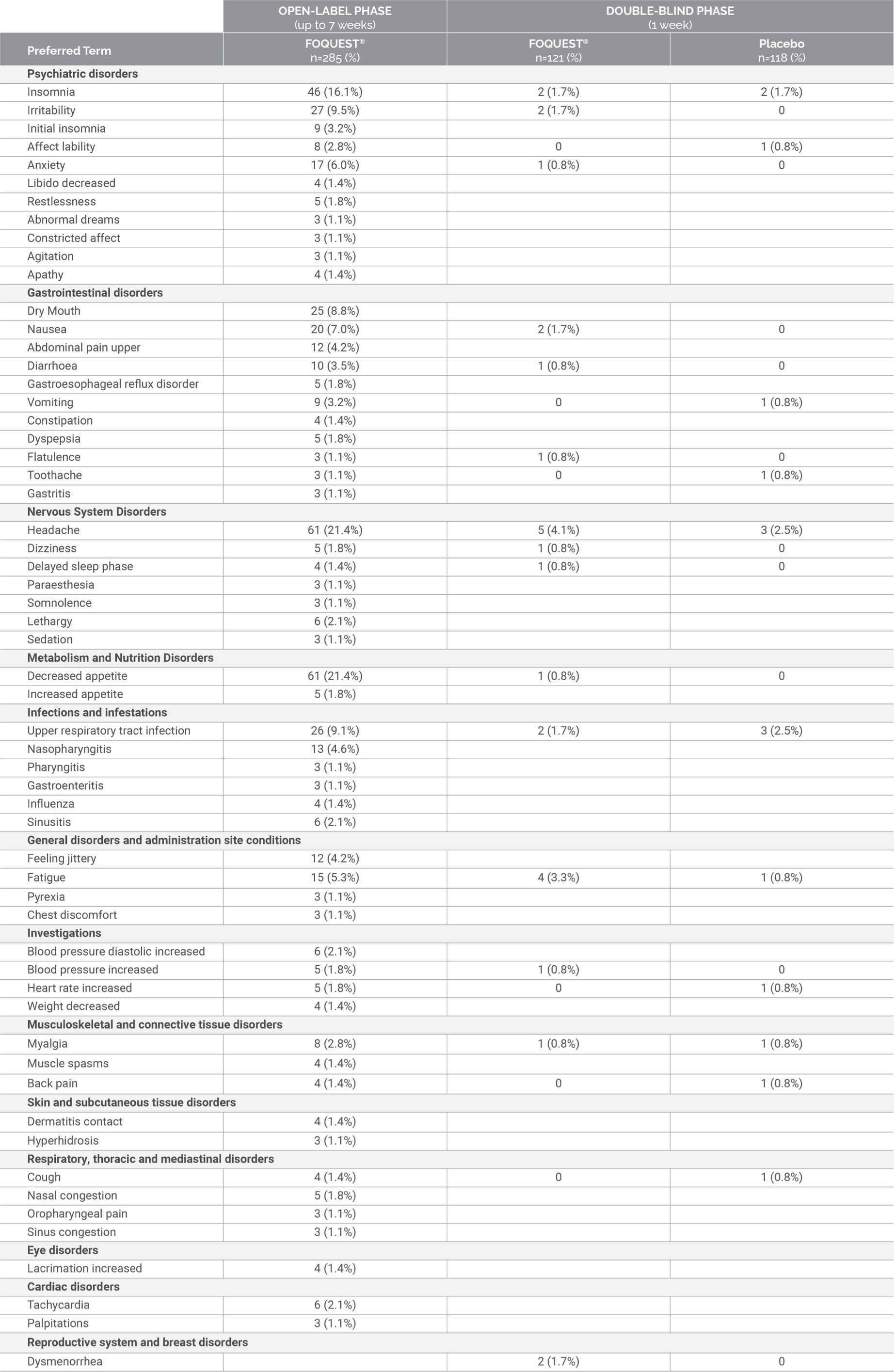
Adapted from the
FOQUEST® product monograph.1
// Open-label phase: All participants started at 25 mg/day. Doses were titrated each week to the next highest
dose according to tolerability: 25, 35, 45, 55, 70, 85 and 100 mg/day. Participants may have returned to a lower
dose if necessary. When optimal dose was reached, participants entered a double-blind, placebo-controlled phase
(half of the participants were randomized to receive placebo while the other half remained on their optimized
dose).