A. For patients already taking methylphenidate, start with FOQUEST® at the next lower strength based on total methylphenidate daily dose. Wait at least 5 days between adjustments, if dose adjustment is warranted. Maximum Daily Dose is 100 mg in adults and 70 mg in children and adolescents 6 to < 18 years.
Please see the FOQUEST® product monograph for complete dosing information.
A. As a general rule for titrating ADHD treatment, CADDRA Guidelines recommend to start low and go slow, but continue to increase the dose until:2
Optimal treatment means that ADHD symptoms have decreased and that there is improvement in general functioning.2 To achieve the optimal FOQUEST® dose, you may slowly increase the dose (each increase should be no less than 5 days apart) to the lowest effective dosage since individual patient response varies widely.1 Maximum daily dose is 100 mg in adults, and 70 mg in children and adolescents 6 to <18 years.
Please see the FOQUEST® product monograph for complete dosing information.
A. In children (6-11 years of age), FOQUEST® was shown to have a duration of action of 13 hours, as measured by SKAMP-C scores (key secondary endpoint).1*
In adults, FOQUEST® was shown to have a duration of action of 16 hours, as measured by PERMP Total score vs. placebo (LS Mean PERMP Total Score at 16h: FOQUEST®: 302.8 vs. placebo, 284.9, p<0.05; key secondary endpoint).1†
* Randomized, double-blind, placebo-controlled, parallel-arm, fixed-dose, multicentre trial measuring the efficacy and safety of FOQUEST® once daily in children aged 6-12 who met the DSM-5 criteria for ADHD. Following a washout period, children (n=156) entered an open-label dose optimization period of up to 6 weeks in which they were given 25 mg once daily in the morning to start, thereafter titrated once weekly from 25 mg to 35 mg to 45 mg to 55 mg to 70 mg to 85 mg FOQUEST® until an optimal dose was reached. Subjects (n=147) then entered 1-week of randomized double-blind treatment with placebo (n=75) or FOQUEST® capsules (n=73) at the optimized dose. (Note, the maximum recommended daily dose of FOQUEST® is 70 mg in children 6 to <18 years of age.1) At the end of the week, investigators evaluated attention and behaviour of subjects in a laboratory classroom setting using the SKAMP rating scale. The primary efficacy endpoint was the difference between FOQUEST® and placebo in mean SKAMP-C score across the entire laboratory classroom trial.A. The PERMP (Permanent Product Measure of Performance) Total score is the sum of the number of math problems attempted plus the number of math problems answered correctly in a 10-minute session with scores ranging from 0-800 with higher scores indicating better performance.1 The PERMP Total score was the primary endpoint in the post-market trial in adults.
A. SKAMP (Swanson, Kotkin, Agler, M-Flynn and Pelham) is a 13-item scale that assesses manifestations of ADHD in a classroom setting. Each item is rated on a 7-point impairment scale: none, slight, mild, moderate, severe, very severe, maximal. A lower score vs. baseline indicates an improvement.1,19 The SKAMP-Combined score was the primary endpoint in the pivotal trial in children.
A. The ADHD-5-RS (ADHD-5-Rating Scale) is an 18-item scale for the assessment of children that incorporates the symptoms of ADHD established in the DSM-5. Items are scored on a 4-point scale from a 0 = not present to 3 = severe, giving a maximum total score of 54. A lower score vs. baseline indicates ADHD symptom improvement.1,3 The ADHD-5-RS score was the primary endpoint in the pivotal trials in adolescents and adults.
A. FOQUEST® has been studied in 1031 ADHD patients across four FOQUEST® clinical trials, including 582 adults, 293 adolescents (12-17 years) and 156 children (6-12 years).
A. Yes, FOQUEST® is currently covered on the:
FOQUEST® is also covered by:
* Official Mark of the Régie de l’assurance maladie du Québec.
A. While both FOQUEST® and BIPHENTIN® are methylphenidate-based medications indicated for the treatment of ADHD in adults and children (6 years of age and older), there are some differences between the two drugs.
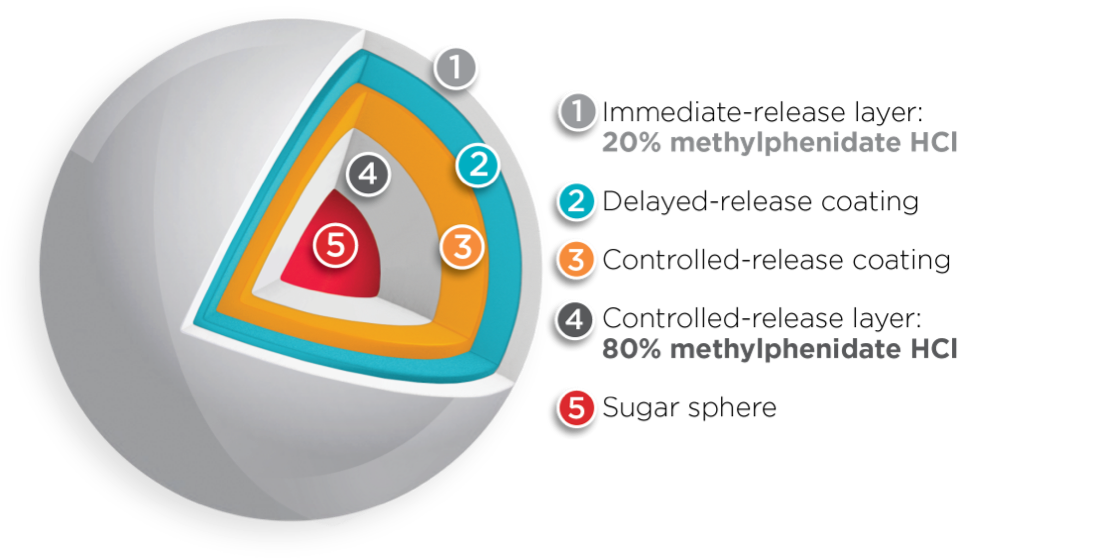
Both BIPHENTIN® and FOQUEST® are uniquely engineered with multi-layer release (MLR®) bead technology1, 5, 20*†
However, the amount of methylphenidate HCl contained within the immediate-release layer and the controlled-release layer differs between the two drugs1,4,6*†
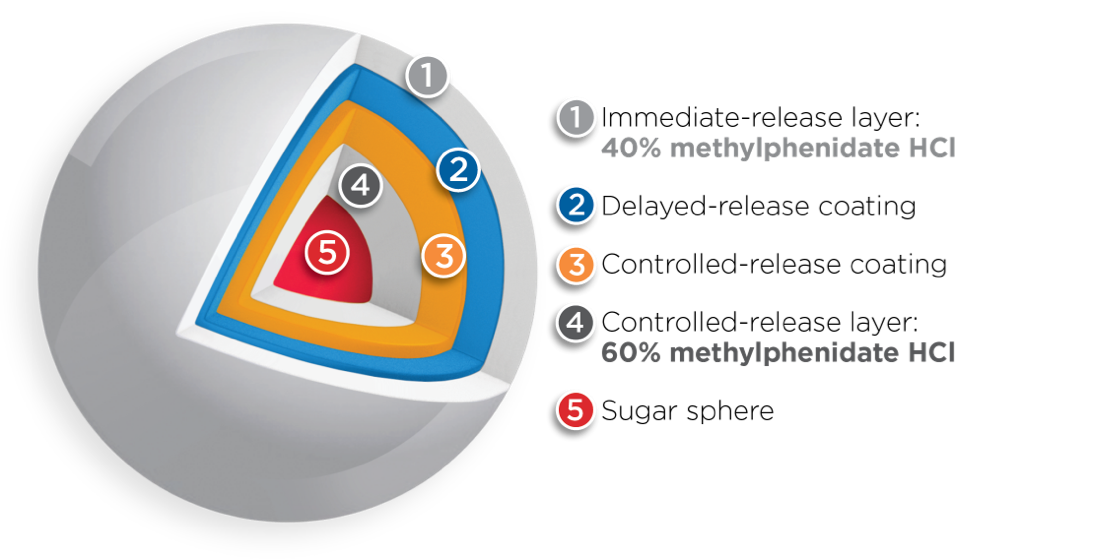
Adapted from Reiz JL, et al.20 * Comparative clinical significance is unknown. † Clinical significance is unknown.
FOQUEST® is a newer* drug, available in Canada since 2017. BIPHENTIN® has been available in Canada since 2006.
BIPHENTIN® (methylphenidate hydrochloride controlled release capsules) is indicated for treatment of Attention-Deficit Hyperactivity Disorder (ADHD) in children (6-11 years of age), adolescents (12-18 years of age), and adults (>18 years of age).
BIPHENTIN® is indicated as an integral part of a total treatment program for ADHD that may include other measures (i.e., psychological, educational, and/or social) for patients with this syndrome. Effectiveness for more than 4 weeks has not been systematically evaluated in placebo-controlled trials. Physicians electing to use BIPHENTIN® for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient.
Click here to consult the product monograph for important information about: