FOQUEST® is taken once daily in the morning, with or without food.1
Tritrate : Slowly, no less than
5 days apart to the lowest
effective dose. Individual response varies widely.
Dose should be adjusted individually and slowly to the lowest effective dose in intervals of no less than 5 days.
FOQUEST® capsules can be swallowed whole (never crushed or chewed), but for those who have a tough time swallowing pills, FOQUEST® can also be sprinkled on applesauce, ice cream, or yogurt. FOQUEST® should not be sprinkled on liquids. The entire mixture should be consumed immediately or within 10 minutes, without chewing.
Patients should rinse mouth with water afterwards to ensure that the entire contents are swallowed

Sprinkle entire contents onto a tablespoon of:
Please see the Product Monograph for complete dosing and administration instructions.
The effect of FOQUEST® might last into the evening, take as soon as possible in the morning to avoid any potential effect on sleep.
FOQUEST® should not be used in patients with symptomatic cardiovascular disease and should generally not be used in patients with known structural cardiac abnormalities.
Patients who are considered to need extended treatment with FOQUEST® should undergo periodic evaluation of their cardiovascular status.
Do not substitute for immediate release methylphenidate tablets or other controlled release methylphenidate products on a milligram for milligram basis because of differing pharmacokinetic profiles.
If a dose increase is warranted, titrate no less than 5 days apart to the lowest effective dose.

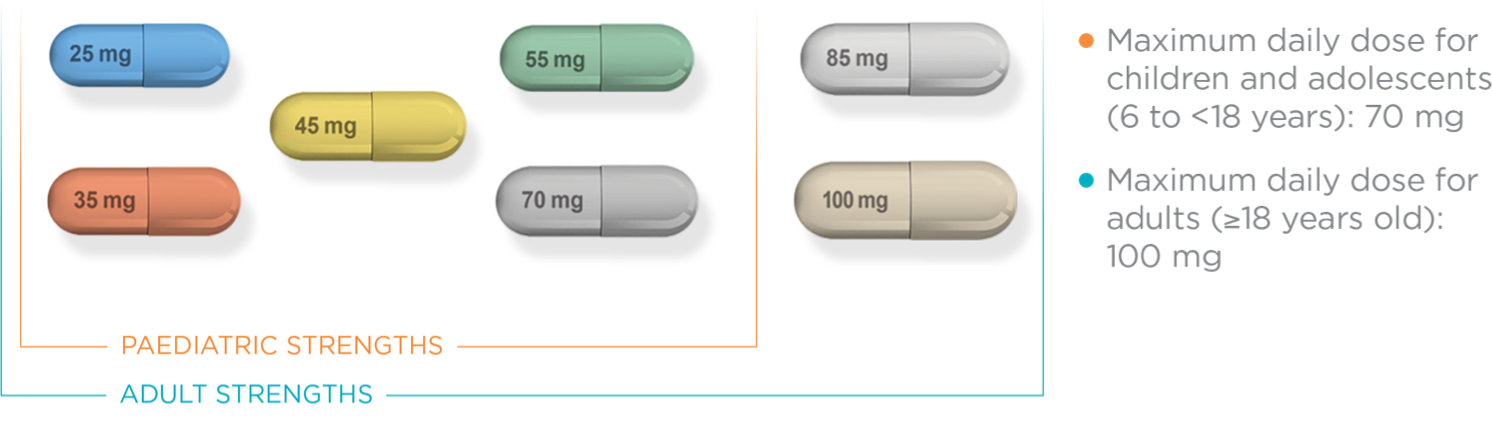
MAXIMUM DAILY
DOSE FOR CHILDREN &
ADOLESCENTS
(6 TO <18 YEARS):
70 mg

MAXIMUM DAILY
DOSE FOR ADULTS
(≥18 YEARS):
100 mg
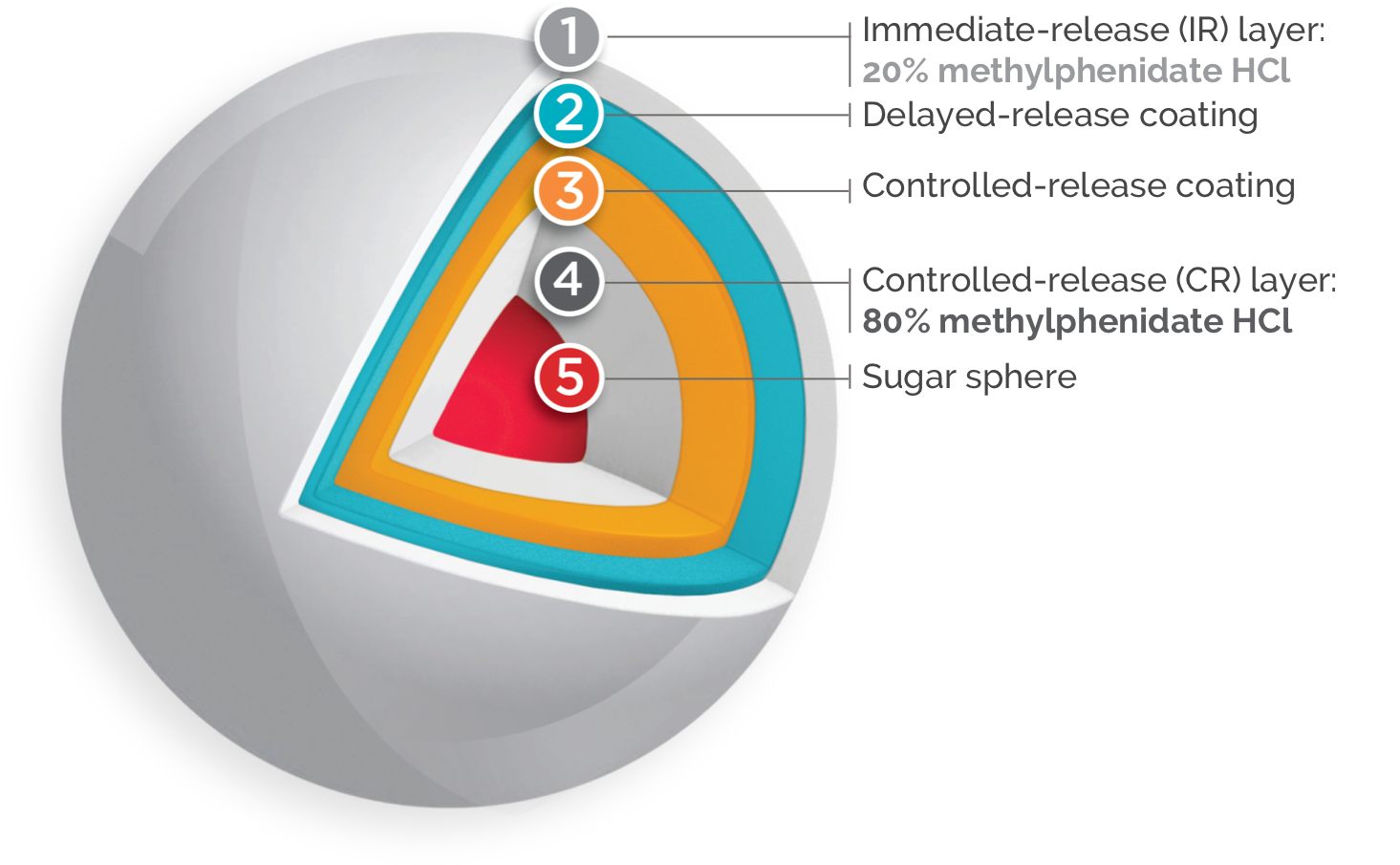
Once-daily FOQUEST® is engineered with Multi-Layer Release (MLR®) Bead Technology1*
FOQUEST® IS DESIGNED TO PROVIDE A BIPHASIC RELEASE OF MPH FROM AN
IMMEDIATE RELEASE LAYER OF THE DRUG, AND DELAYED CONTROLLED RELEASE
LAYERS OF THE
DRUG.1

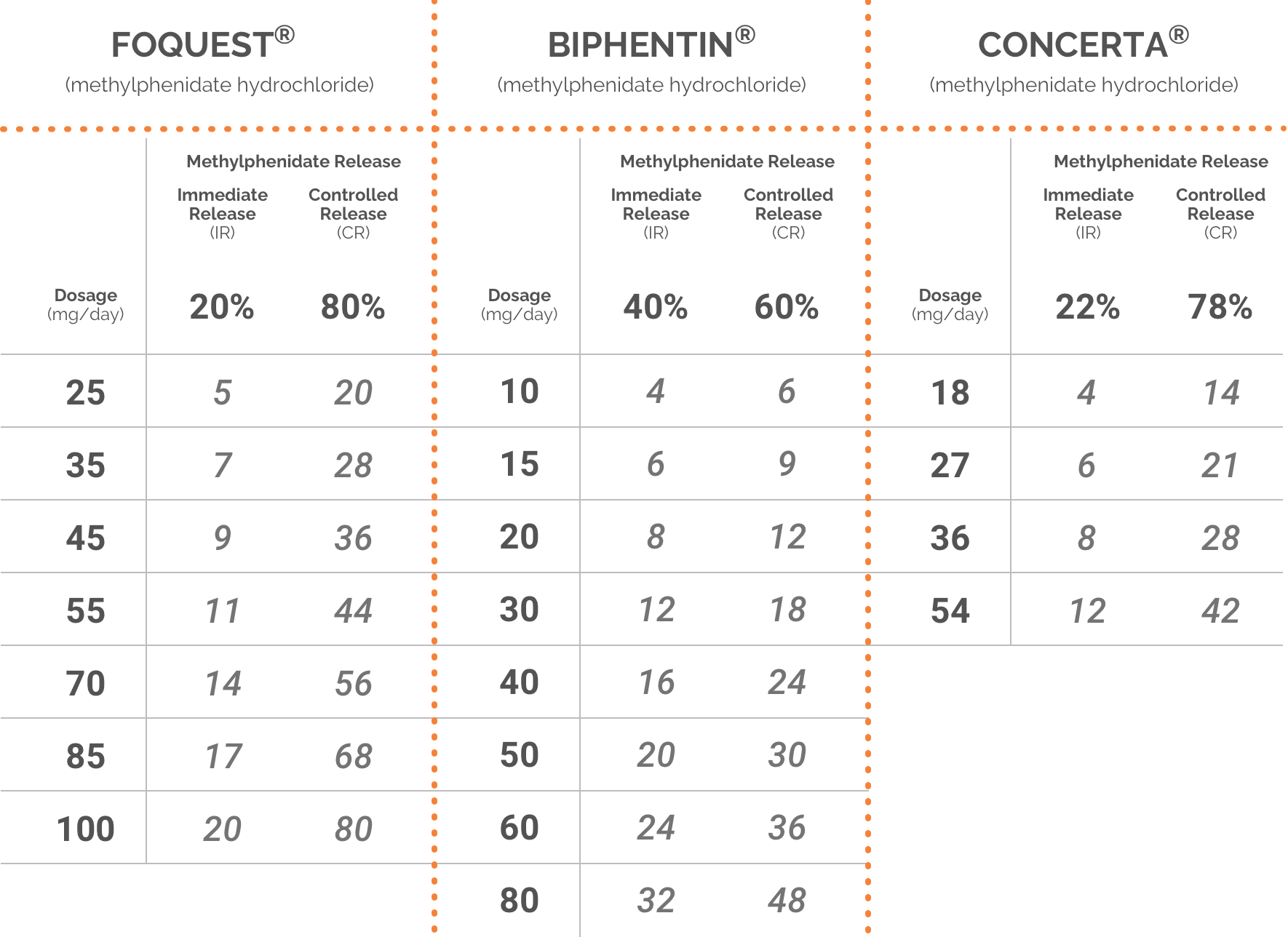
Adapted from the FOQUEST®, BIPHENTIN®, and CONCERTA® product monographs.1,5,6
Please consult respective product monographs for complete dosing information.
FOQUEST® (methylphenidate hydrochloride controlled release capsules) is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in patients ≥6 years of age.
CONCERTA® (methylphenidate hydrochloride) is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in children (6-12 years of age), adolescents (13-18 years of age), and adults (>18 years of age).
BIPHENTIN® (methylphenidate hydrochloride controlled release capsules) is indicated for treatment of Attention Deficit Hyperactivity Disorder (ADHD) in children (6-11 years of age), adolescents (12-18 years of age), and adults (>18 years of age).
BIPHENTIN® is indicated as an integral part of a total treatment program for ADHD that may include other measures (i.e., psychological, educational, and/or social) for patients with this syndrome. Effectiveness for more than 4 weeks has not been systematically evaluated in placebo-controlled trials. Physicians electing to use Biphentin® for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient.
Click here to consult the product monograph for important information about:
* Comparative clinical significance is unknown